Tenofovir Disoproxil Fumarate
This page provides detailed information about Tenofovir Disoproxil Fumarate, covering its indication, dosage, administration, mechanism of action, related brands with strengths, warnings, and common side effects.
Context and Approval Date
Tenofovir Disoproxil Fumarate, an antiviral medication, received approval for its medical application on October 26, 2001.
Mechanism of Action of Tenofovir Disoproxil Fumarate
Tenofovir Disoproxil Fumarate Tablet is classified as an antiretroviral medication, specifically a nucleoside reverse transcriptase inhibitor. Its mechanism involves boosting the CD4 T cell count and activating the immune system to combat infections. Consequently, it lowers the HIV viral load in the bloodstream and mitigates the severity of HIV infection.
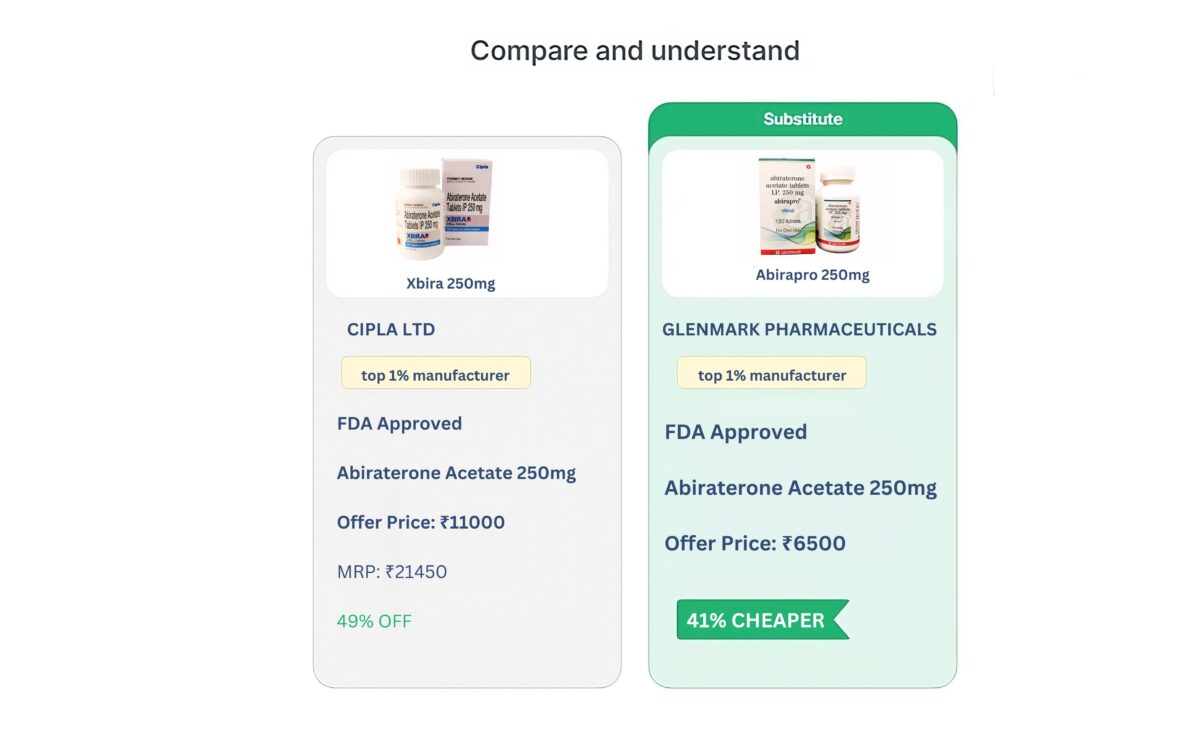
All Substitutes
View ALL| Product | Packaging Size | Company | Price |
|---|---|---|---|
| Ricovir 300mg tablet | 30 tablets | Mylan Pharmaceuticals | INR 750 |
| Teravir 300mg tablet | 30 tablets | Natco Pharma | INR 890 |
| Tenohep 300mg tablet | 30 tablets | Zydus Cadila | INR 950 |
| Tenvir 300mg tablet | 30 tablets | CIPLA Ltd | INR 1050 |
| Tenof 300mg tablet | 30 tablets | Hetero Healthcare | INR 1030 |
| Samhep TDF 300mg tablet | 30 tablets | Samarth Life Sciences | INR 1450 |
Uses of Tenofovir Disoproxil Fumarate
Tenofovir Disoproxil Fumarate is formulated to address human immunodeficiency virus (HIV-1) and hepatitis B virus (HBV) infections in individuals aged 2 years and older, both adults and children.
Tenoforvir Disoproxil Fumarate administration guidelines and available dosage options
Tenofovir Disoproxil Fumarate is supplied in the form of film-coated tablets, each containing 300mg of the active ingredient. The typical prescribed dosage is one tablet daily, with or without food. For adults and children weighing over 35kg, the standard daily dose is 300mg. In the case of children weighing between 17kg and 35kg, the precise dosage will be determined by the physician, based on the child's weight.
Information about Tenofovir Disoproxil Fumarate
Tenofovir disoproxil fumarate Uses
The treatment for chronic hepatitis B virus (HBV) infection is tenofovir disoproxil fumarate.How Tenofovir disoproxil fumarate works
Tenofovir disoproxil fumarate is an antiviral medication that inhibits the replication of viruses within human cells. By preventing the production of new viruses, it effectively combats the infection and promotes clearance.Common side effects of Tenofovir disoproxil fumarate
Diarrhea, Nausea, Rash, Weakness, Vomiting, Fatigue, Abdominal pain, Increased transaminase level in the blood, Decreased phosphate level in the blood, Pain, Dizziness, Depression, Insomnia (difficulty sleeping), Fever, and Itching.1. You have been prescribed Tenofovir disoproxil fumarate for the treatment of chronic hepatitis B virus (HBV) infection.
In combination with other medicines, it is used for the treatment of HIV infections.
2. Take it with food, as this increases the absorption of the medicine into the body.
3. Tenofovir disoproxil fumarate may cause dizziness or sleepiness. Do not drive or do anything requiring concentration until you know how it affects you.
4. You may still develop infections or other illnesses associated with viral infection while taking this medication. You can also pass on HIV or HBV to others. Don't share needles or personal items that can have blood or body fluids on them.
5. During treatment and for at least six months after stopping this medicine, regular blood tests are needed to monitor your liver function, level of hepatitis B virus and blood cells in your blood.
2. Take it with food, as this increases the absorption of the medicine into the body.
3. Tenofovir disoproxil fumarate may cause dizziness or sleepiness. Do not drive or do anything requiring concentration until you know how it affects you.
4. You may still develop infections or other illnesses associated with viral infection while taking this medication. You can also pass on HIV or HBV to others. Don't share needles or personal items that can have blood or body fluids on them.
5. During treatment and for at least six months after stopping this medicine, regular blood tests are needed to monitor your liver function, level of hepatitis B virus and blood cells in your blood.
Warning, Precautions, and Side Effects Associated with Tenofovir disoproxil fumarate
Warning
If you have experienced hypersensitivity reactions to Tenofovir disoproxil fumarate or any of its ingredients, avoid taking this medication. During Tenofovir Disoproxil Fumarate Tablet treatment, your physician may conduct regular monitoring of your blood counts, bone density, liver, and kidney function to avert potential complications. Inform your doctor about any history of heart failure, pancreatitis, bone disorders, fractures, or liver and kidney issues.Precautions
If you can have kids, think about using birth control to avoid getting pregnant unexpectedly. If you're pregnant or might be pregnant, your doctor may recommend a different treatment because it's not safe to use this medicine early in pregnancy. We don't suggest breastfeeding because it could pass HIV from mom to baby.Side effects
The common side effects that are likely to occur while you are on treatment with Tenofovir Disoproxil Fumarate Tablet are pain, depression, nausea, vomiting, weakness, headache, diarrhea, rash, stomach pain, sleeping problems, dizziness, itching, and fever. Some serious adverse reactions include bone loss, liver problems, kidney injury, immune reconstitution syndrome, and lactic acidosis. If you experience any allergic reactions after taking this tablet, report them to your doctor immediately.Word of advice
Tenofovir disoproxil fumarate may cause dizziness and fatigue. Therefore, it's best to refrain from driving or operating machinery while undergoing this treatment. Never discontinue Tenofovir Disoproxil Fumarate Tablet without consulting your healthcare provider.FAQ - Tenofovir Disoproxil Fumarate
1How much time does it typically take for Tenofovir Disoproxil Fumarate to start showing its effects?
Tenofovir Disoproxil Fumarate usually requires around 4 weeks to lower the viral load in the bloodstream and show its effectiveness.
2Is it safe to drink alcohol while taking Tenofovir Disoproxil Fumarate Tablet?
The risk of liver damage and dysfunction increases with Tenofovir Disoproxil Fumarate Tablet. Drinking alcohol during this treatment further increases the risk of liver injury.
3What lab tests are necessary before starting this treatment?
If you're a teenager or adult who could become pregnant, your healthcare provider will conduct a pregnancy test before recommending this treatment. Other important tests include screening for HBV infection, checking serum creatinine levels, estimating creatinine clearance, and assessing urine glucose and protein levels.
4What are the signs that indicate you might be allergic to Dolutegravir, Lamivudine, or Tenofovir disoproxil fumarate?
Keep an eye out for things like a bad rash, a rash with a fever, sore muscles, feeling super tired, a puffy face, trouble breathing, blisters, or your skin peeling. If you have any of these, immediately contact your doctor for assistance.
5What are some of the long-term side effects of this medication?
This medicine can cause severe side effects such as hepatitis B worsening, bone weakening, kidney damage, immune reconstitution syndrome, liver problems, and allergic reactions.
6What are the symptoms of lactic acidosis?
The main signs of lactic acidosis include unusual weakness, tiredness, muscle aches, difficulty breathing, stomach discomfort, nausea, vomiting, dizziness, feeling faint, an irregular heartbeat, and feeling cold, especially in your arms and legs.
References
1. Gilead Sciences, Inc., U.S. Food and Drug Administration, [Revised on Apr 2019] [Accessed on 17th Sep 2022],https://www.accessdata.fda.gov/
drugsatfda_docs/pepfar/210787PI.pdf
2. KD Tripathi, Antiviral Drugs (Anti-retrovirus), Essentials of Medical Pharmacology, 8th Edition, 2019, 860-872.
drugsatfda_docs/pepfar/210787PI.pdf
2. KD Tripathi, Antiviral Drugs (Anti-retrovirus), Essentials of Medical Pharmacology, 8th Edition, 2019, 860-872.