Bevacizumab
This page provides detailed information about bevacizumab, covering its indication, dosage, administration, mechanism of action, related brands with strengths, warnings, and common side effects.
Context and Approval Date
Bevacizumab, the first antiangiogenic agent, was approved for medical use in 2004. This drug blocks the formation of new blood vessels, thereby inhibiting tumor growth.
Mechanism of Action of Bevacizumab
Bevacizumab injection is a biologic agent used in targeted cancer therapy. Classified as a monoclonal antibody and VEGF inhibitor, it binds selectively to VEGF-A, a protein involved in the formation of blood vessels that supply nutrients and oxygen to tumors. By blocking VEGF-A, bevacizumab inhibits tumor growth. People commonly use it in combination with other chemotherapy agents.
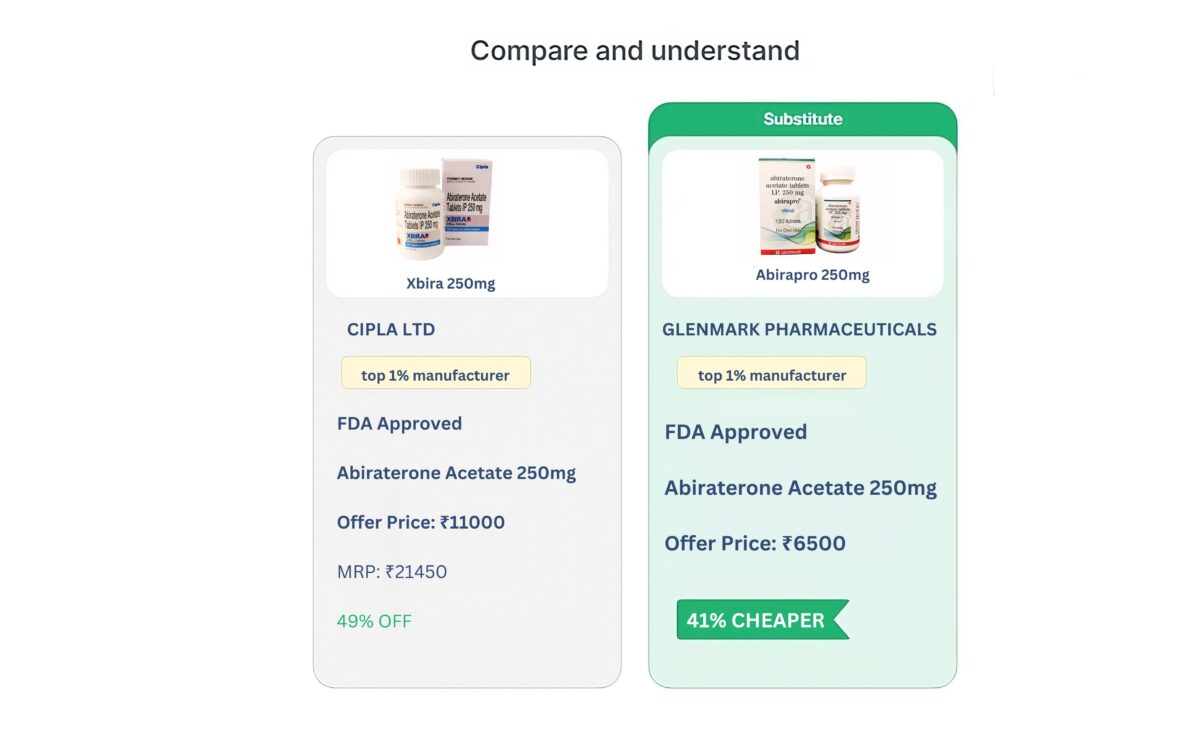
All Substitues
View All
| Product | Packaging Size | Company | Price |
|---|---|---|---|
| Bryxta 400mg Injection | 1 Vial | Zydus Calida | INR 12,000 |
| Bevnexxa 400mg Injection | 1 Vial | Celon Labortories Ltd | INR 10,000 |
| Bevaza 400mg Injection | 1 Vial | Lupin Ltd | INR 9500 |
| Bevacirel 400mg Injection | 1 Vial | Reliance Life Sciences Pvt Ltd | INR 14000 |
| Cizumab 400mg Injection | 1 Vial | Hetero Healthcare | INR 7000 |
| Bryxta 100mg Injection | 1 Vial | Zydus Calida | INR 5000 |
Uses of Bevacizumab
Bevacizumab injection is designed to treat solid tumors by administering it directly into the bloodstream. Various primary and advanced-stage cancers, such as metastatic breast cancer, metastatic cervical cancer, glioblastoma multiforme (brain cancer), advanced kidney cancer, metastatic colorectal cancer, advanced non-small-cell lung cancer, hepatocellular carcinoma (liver cancer), and cancers of the ovary, cervix, fallopian tube, or primary peritoneal cancer, have received approval for its treatment.
Bevacizumab administration guidelines and available dosage options
A healthcare professional will administer a Bevacizumab injection as an intravenous infusion. The healthcare professional administers the initial dose over 90 minutes, and if well tolerated, administers subsequent infusions over 30 minutes. The patient's response to the therapy determines the number of infusions.
Based on the patient's height, weight, body surface area, and the type of cancer they are treating, the oncologist will determine the appropriate dose of Bevacizumab. This injection is available in two dosages: 100 mg and 400 mg. Specifically, a 4 ml vial contains 100 mg of Bevacizumab, while a 16 ml vial contains 400 mg of Bevacizumab.
Based on the patient's height, weight, body surface area, and the type of cancer they are treating, the oncologist will determine the appropriate dose of Bevacizumab. This injection is available in two dosages: 100 mg and 400 mg. Specifically, a 4 ml vial contains 100 mg of Bevacizumab, while a 16 ml vial contains 400 mg of Bevacizumab.
Information about Bevacizumab
Bevacizumab uses
Bevacizumab is used in the treatment of cancer of the colon and rectum, ovarian cancer, cervical cancer, kidney cancer, brain tumors, and non-small cell lung cancer.How bevacizumab works
Bevacizumab 400 injection is an anti-angiogenic medication. It works by inhibiting a protein called vascular endothelial growth factor (VEGF), which prevents the formation of blood vessels that supply oxygen and nutrients to cancer cells.Common side effects of bevacizumab
Exfoliative dermatitis, High blood pressure, Nosebleeds, Protein in urine, Decreased appetite, Electrolyte imbalance, Peripheral sensory neuropathy, Breathlessness, Thromboembolism, Wound healing complications, Ovarian failure, Decreased white blood cell count (lymphocytes), Low blood platelets, Mucosal inflammation, Abscess, Cellulitis, Hypersensitivity, Rectovaginal fistula.1. Bevacizumab is an effective first-line option when used together with other medicines for certain types of cancers, such as colorectal, lung, cervical, and kidney cancers.
2. Bevacizumab is given as an infusion. Your doctor or nurse will monitor you for signs of an infusion reaction, such as high blood pressure and trouble breathing.
3. It can impair your wound healing ability. Inform your doctor that you are taking this medication before undergoing any surgical procedure.
4. It can increase the risk of bleeding. If you notice any unusual bleeding or bleeding that doesn't stop easily, please inform your doctor.
5. Your doctor may regularly check your blood pressure and levels of protein in your urine while you are on Bevacizumab.
2. Bevacizumab is given as an infusion. Your doctor or nurse will monitor you for signs of an infusion reaction, such as high blood pressure and trouble breathing.
3. It can impair your wound healing ability. Inform your doctor that you are taking this medication before undergoing any surgical procedure.
4. It can increase the risk of bleeding. If you notice any unusual bleeding or bleeding that doesn't stop easily, please inform your doctor.
5. Your doctor may regularly check your blood pressure and levels of protein in your urine while you are on Bevacizumab.
Warning, Precautions, and Side Effects Associated with Bevacizumab
Warning
Contact your doctor immediately if you experience shortness of breath, a fever over 100°F, or severe bleeding. During Bevacizumab injection treatment, your doctor may periodically monitor your white blood cell counts, platelets, blood sugar, electrolytes, and kidney parameters to prevent serious complications. Inform your doctor if you have a history of heart failure. We do not recommend taking this medication within 28 days before or after surgery, as it may delay wound healing. Seek immediate medical attention if you experience any visual disturbances.Precaution
The delayed effects of taking Bevacizumab injections can include menstrual irregularities, ovarian failure, and impaired fertility in women. Both men and women should use proper contraceptive methods if they plan to become pregnant in the near future. We recommend continuing birth control for at least 6 months after completing the treatment. Avoid breastfeeding during treatment, as bevacizumab can pass into breast milk and potentially impact the baby's growth and development.Side Effects
Common side effects during Bevacizumab injection treatment may include numbness in the feet, extreme fatigue, high blood pressure, dry mouth, bleeding complications, stomach perforations, skin blisters, increased pulse rate, darkened urine, mouth sores, blood clots in vessels, and muscle and joint pain. If you experience any allergic reactions, such as excessive sweating, chest pain, or tremors, report them to your doctor immediately.FAQ - Bevacizumab
1Is Bevacizumab injection used as a standalone treatment for cancer?
Bevacizumab injection is a recombinant monoclonal antibody classified under immunotherapy for cancer treatment. You can administer it as a monotherapy or in combination with chemotherapy or other immunotherapy drugs to enhance overall survival rates.
2Bevacizumab injection Is safe for women to use?
For women of reproductive potential receiving Bevacizumab injections, it is essential to discuss effective birth control methods with your doctor during treatment. Contraceptive methods should be continued for at least 6 months after the treatment is completed, as this medication may impair fertility.
3 Is it safe to undergo surgery while receiving a Bevacizumab injection?
Bevacizumab injections are linked to an increased risk of bleeding complications and poor wound healing. Therefore, we recommend avoiding surgery scheduling and maintaining a 28-day interval before or after any surgical procedure.
4 Do the side effects of Bevacizumab injection last for a chronic period?
Bevacizumab injection-related side effects persist until the drug concentration remains within the body. Our body retains this medicine for nearly 4 months after the final dose.
5 How is Bevacizumab injection administered?
We administer Bevacizumab injection intravenously through infusion, measuring the drug's amount according to the patient's body weight, height, and age. We will administer the first dose for 90 minutes, and if the patient tolerates it well, we can reduce the infusion time to 30 minutes.
References
1. https://www.cancer.gov/about-cancer/treatment/drugs/bevacizumab
2. https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/cancer-drugs/drugs/bevacizumab
3. https://www.gene.com/download/pdf/
avastin_prescribing.pdf
4. https://www.medicines.org.uk/emc/files
/pil.3885.pdf
5. https://chemocare.com/chemotherapy/
drug-info/bevacizumab.aspx
2. https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/cancer-drugs/drugs/bevacizumab
3. https://www.gene.com/download/pdf/
avastin_prescribing.pdf
4. https://www.medicines.org.uk/emc/files
/pil.3885.pdf
5. https://chemocare.com/chemotherapy/
drug-info/bevacizumab.aspx