Imatinib
This page provides brief information on the medication imatinib, covering its approved uses, recommended dosage and administration, mode of action, associated brand names and strengths, warnings, and side effects.
Context and Approval Date
On May 10, 2001, the FDA approved imatinib for use as a targeted anticancer treatment drug.
Mechanism of Action of Imatinib
Imatinib, a biological agent from the tyrosine kinase inhibitor class, serves as a targeted pharmacological therapy for cancer treatment. By interfering with cellular signaling pathways, it stops the spread and multiplication of cancer cells by targeting the protein BCR-ABL tyrosine kinase.
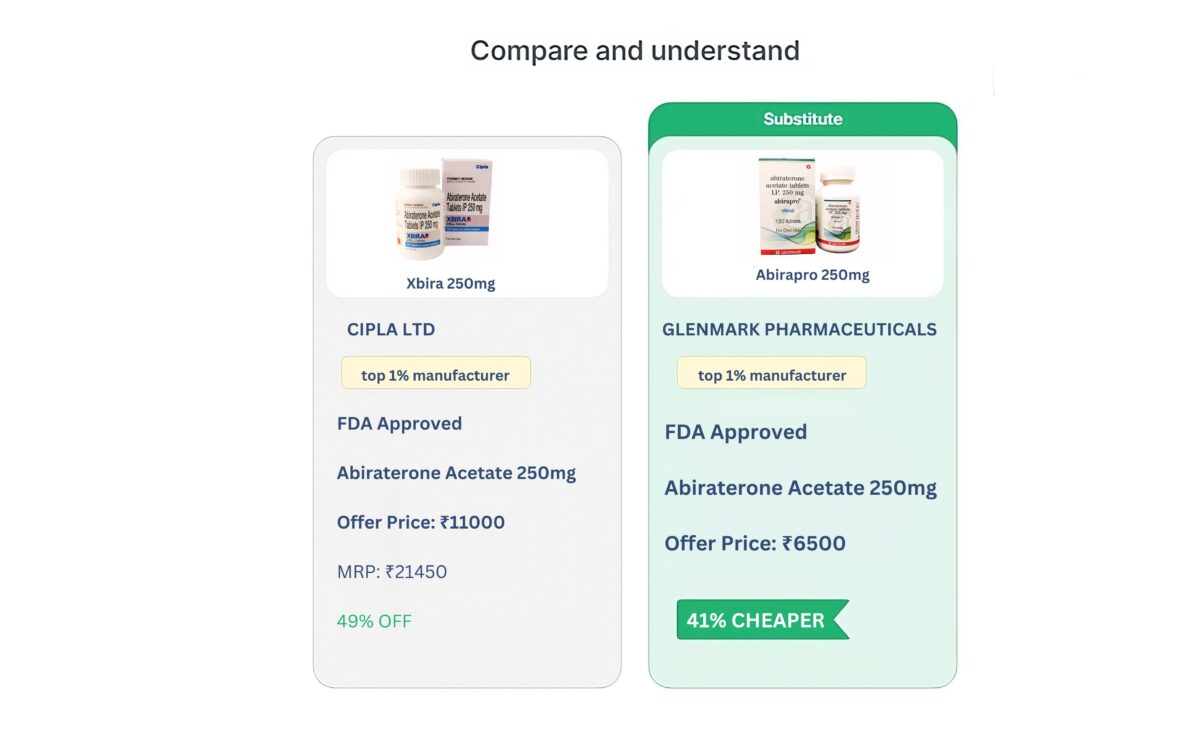
All Substitutes
View All| Product | Packaging Size | Company | Price |
|---|---|---|---|
| Imatinib 400mg tablet | 5 x 3 x 10 tablets | Natco Pharma | INR 810 |
| Mytinib 400mg tablet | 3 x 10 tablets | Mylan Pharmaceuticals | INR 550 |
| Apotinib 400mg tablet | 3 x 10 tablets | BDR Pharmaceuticals | INR 580 |
| Imat 400mg tablet | 10 tablets | Zydus Cadlia | INR 660 |
| Samitib 400mg tablet | 10 tablets | Samarth Life Sciences | INR 750 |
| Imatero 400mg tablet | 10 tablets | Hetero Healthcare | INR 730 |
| Imatib 400mg tablet | 10 tablets | CIPLA Ltd | INR 750 |
| Veenat 400mg tablet | 10 tablets | Natco Pharma Ltd | INR 810 |
| Lupinib 400mg tablet | 10 tablets | Lupin Ltd | INR 1230 |
Uses of Imatinib
Imatinib can help with a lot of different conditions, such as cancers of the gastrointestinal stromal tract (a type of stomach cancer), the skin cancer Dermatofibrosarcoma protuberans, Philadelphia chromosome-positive chronic myeloid leukemia (CML) in blast crises, accelerated and chronic phases, and Philadelphia chromosome-acute lymphoblastic leukemia.
Dasatinib administration guidelines and available dosage options
Imatinib is offered in tablet and capsule forms with doses of 100mg and 400mg. Your healthcare provider will determine the appropriate dosage based on your condition. Take Imatinib tablets or capsules with a large amount of water. If swallowing is difficult, consider dissolving the tablet or capsule contents in water or apple juice before consumption.
information about Imatinib
Treatment for blood cancer (chronic myeloid leukemia), blood cancer (acute lymphocytic leukemia), and gastrointestinal stromal tumors involves imatinib mesylate.
Imatinib mesylate functions as an anti-cancer medication targeting the protein enzyme bcr-abl tyrosine kinase, which is responsible for the aberrant growth and proliferation of cancer cells. By inhibiting proliferation and inducing apoptosis (planned cell death) in bcr-abl-positive cells (cancer cells), this medicine effectively halts or slows the spread of cancer.
Edema (swelling), Fatigue, Rash, Fever, Hair loss, Insomnia (difficulty sleeping), Joint pain, Muscle pain, Weight gain, Night sweats, Itching, Gastrointestinal toxicity, Blood cell abnormalities, Liver toxicity, Renal toxicity, Nasopharyngitis (inflammation of the throat and nasal passages) is an upper respiratory tract infection.
Warning, Precautions, and Side Effects Associated with Imatinib
Warning
If you experience symptoms like shortness of breath, rapid heartbeat, fever, or severe bleeding, contact your doctor immediately. During Imatinib treatment, your doctor may periodically monitor various parameters, including complete blood counts, blood sugar levels, electrolytes, thyroid function, and kidney function, to mitigate potential complications. If your doctor diagnoses you with conditions like diabetes, hypertension, heart problems, liver issues, skin disorders, kidney problems, or blood disorders, let them know. It is advisable to avoid undergoing surgery or dental procedures while on Imatinib treatment due to the elevated risk of grade ¾ hemorrhage. Prior to any surgical or dental procedures, inform your physician. Get immediate medical attention if you experience itching, yellowing of the eyes or skin, skin rashes, or pain or discomfort in the right upper stomach area.Precautions
Imatinib can be harmful to an unborn baby. Prior to starting this treatment, inform your healthcare provider if you are pregnant or planning to conceive. Both men and women should use effective contraceptive methods during Imatinib treatment and for 14 days after the final dose. Stop breastfeeding while undergoing treatment and for one month following the last dose, as Imatinib can affect the baby's growth and development by transferring through breast milk.Side effects
The common side effects that are likely to occur while you are on treatment with Imatinib are edema, nausea, vomiting, muscle cramps, musculoskeletal pain, diarrhea, rash, cough, sinusitis, bone pain, constipation, and tiredness. Some serious side effects of Imatinib treatment include heart problems, hypothyroidism, severe skin reactions, bleeding, liver impairment, kidney impairment, and stomach problems. If you experience a severe headache, lightheadedness, confusion, dizziness, or severe abdominal pain, report it to your doctor immediately.Word of advice
Imatinib may cause constipation. It's crucial to stay hydrated and stick to a balanced diet. Notify your healthcare provider if you observe unusual weight gain. If you experience dizziness, blurred vision, or fatigue during Imatinib treatment, refrain from driving or operating heavy machinery.FAQ - Imatinib
1What are the common side effects of imatinib?
Common side effects usually experienced during Imatinib treatment include edema, nausea, vomiting, muscle cramps, musculoskeletal pain, diarrhea, rash, cough, sinusitis, bone pain, constipation, and fatigue.
2Is Imatinib safe to use during pregnancy?
Imatinib may be harmful to an unborn infant. If you are pregnant or plan to have a baby, notify your healthcare practitioner before starting this treatment.
3Is it safe to have surgery while undergoing treatment with Imatinib?
Imatinib is associated with an increased risk of bleeding problems and delayed wound healing. As a result, we advise against scheduling surgery while receiving Imatinib medication. Consult your doctor before considering surgery or dental procedures.
4How long can Imatinib be taken?
Imatinib can be taken for as long as your doctor prescribes. Many healthcare practitioners give Imatinib for three years to individuals who are at high risk of cancer recurrence.
5What medications or substances should be avoided while taking Imatinib?
It is not recommended to take warfarin, heparin, erythromycin, or phenytoin when using Imatinib. Inform your healthcare practitioner if you are taking any prescription or over-the-counter medications, vitamins, or herbal supplements.
6When should you inform your doctor?
If you develop unusual weight gain, loss of appetite, bleeding, bruising, itching, yellowing of the skin and eyes, shortness of breath, stomach pain, dizziness, irregular heartbeat, or disorientation, contact your doctor immediately.
7How should Imatinib be stored?
Imatinib pills and capsules should be stored at room temperature and out of children's reach. Protect against moisture.
References
1. Novartis Pharmaceuticals Corporation, US Food & Drug Administration, [Revised on Aug 2022] [Accessed on 10th Sep 2022], https://www.novartis.com/us-en/sites/novartis_us/files/gleevec_tabs.pdf
2. KD Tripathi, Anticancer Drugs, Essentials of Medical Pharmacology, 8th Edition, 2019, 915-936.
3. Anton Wellstein, Giuseppe Giaccone, Michael B. Atkins, and Edward A. Sausville, Goodman & Gilman’s Pharmacological Basis of Therapeutics, Pathway-Targeted Therapies: Monoclonal Antibodies, Protein Kinase Inhibitors, and Various Small Molecules, 13th Edition, 2018, 1203-1236.
2. KD Tripathi, Anticancer Drugs, Essentials of Medical Pharmacology, 8th Edition, 2019, 915-936.
3. Anton Wellstein, Giuseppe Giaccone, Michael B. Atkins, and Edward A. Sausville, Goodman & Gilman’s Pharmacological Basis of Therapeutics, Pathway-Targeted Therapies: Monoclonal Antibodies, Protein Kinase Inhibitors, and Various Small Molecules, 13th Edition, 2018, 1203-1236.