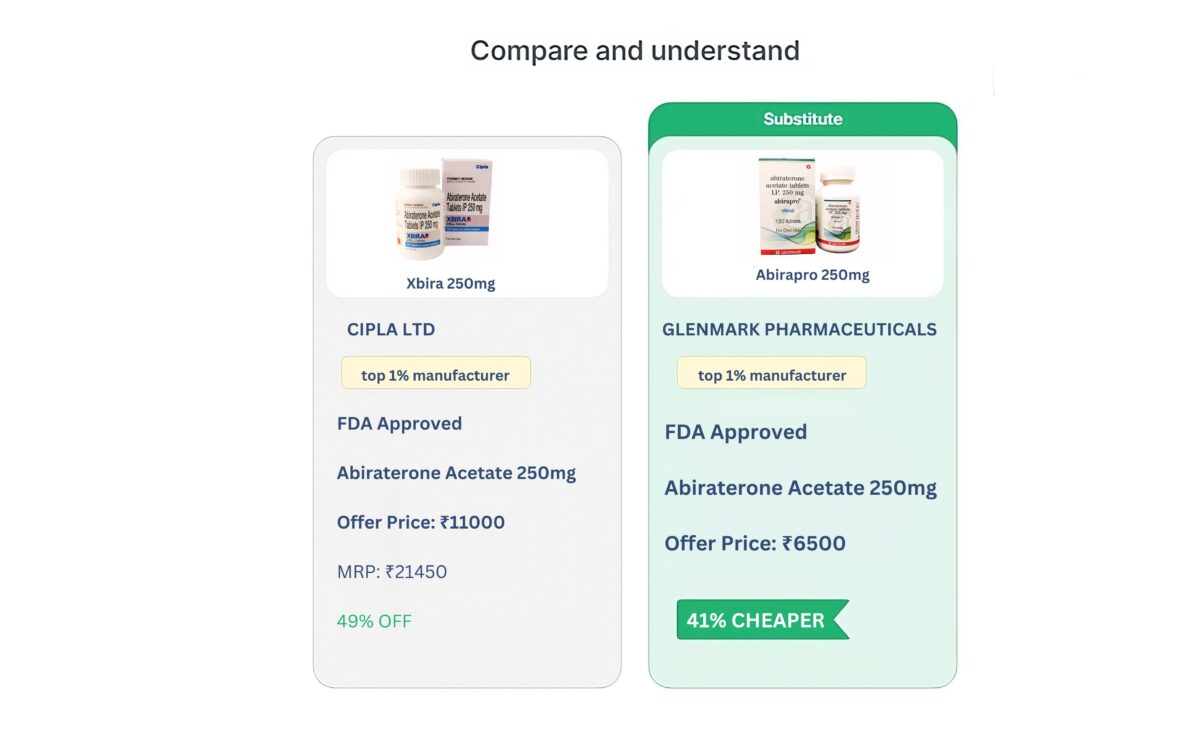
Nimotuzumab
This page provides a summary of the drug nimotuzumab, including its indications, dosage and administration guidelines, mechanism of action, related brands and their strengths, as well as warnings and common side effects.
Context and Approval Date
Nimotuzumab is a monoclonal antibody containing the active ingredient Nimotuzumab, used in cancer treatment. It is approved for treating head and neck cancer, as well as specific types of brain cancer. This medication is often used in combination with other cancer treatments, such as chemotherapy and radiation therapy.
While there are no absolute contraindications to Nimotuzumab, certain precautions and potential contraindications should be considered. For instance, patients with a history of severe allergic reactions or hypersensitivity to Nimotuzumab or its components should avoid this medication. Additionally, patients with conditions such as severe liver or kidney disease may require close monitoring or dosage adjustments.
While there are no absolute contraindications to Nimotuzumab, certain precautions and potential contraindications should be considered. For instance, patients with a history of severe allergic reactions or hypersensitivity to Nimotuzumab or its components should avoid this medication. Additionally, patients with conditions such as severe liver or kidney disease may require close monitoring or dosage adjustments.
Mechanism of Action of Nimotuzumab
Nimotuzumab works by targeting and binding to the epidermal growth factor receptor (EGFR) present on many cancer cells. This action helps block the growth and spread of cancer cells, ultimately leading to better outcomes for cancer patients. One of the key benefits of this medication is its targeted therapy approach, which specifically targets cancer cells while minimizing harm to healthy cells. This can help reduce side effects and improve the overall quality of life for patients undergoing cancer treatment.
Uses of Nimotuzumab
Nimotuzumab is used to treat various types of cancer, including head and neck cancer, as well as certain brain cancers like glioma and glioblastoma. It is often administered alongside other cancer treatments, such as chemotherapy and radiation therapy, to enhance overall and disease-free survival outcomes.
Nimotuzumab administration guidelines and available dosage options
Nimotuzumab is an injectable medication administered by a healthcare professional, usually through an intravenous injection. The dosage and frequency depend on the type of cancer being treated, the patient's overall health, and any other medications being taken. If a dose is missed, contact your healthcare provider promptly. They may suggest rescheduling the next dose or adjusting the dosing schedule. Do not take a double dose to compensate for a missed one.
Information about Nimotuzumab
Nimotuzumab Uses
Nimotuzumab is used in the treatment of head and neck cancer, as well as colon and rectal cancer.How Nimotuzumab works
Nimotuzumab is a monoclonal antibody that selectively binds to receptors on cancer cells, marking them for destruction by the body's immune system.Common side effects of Nimotuzumab
Weakness, Vomiting, Dizziness, Blood in urine, Diarrhea1. Nimotuzumab is given as an injection or infusion under the supervision of a doctor only.
2. Do not skip any doses and complete the course as suggested by your doctor.
3. Use an effective method of birth control to avoid pregnancy while taking this medication.
4. Your doctor may get regular blood tests done to monitor your blood cell count (CBC) and electrolyte levels. liver and kidney function.
5. It is advisable to drink plenty of water to stay hydrated while taking this medicine.
6. Inform your doctor if you experience chills, fever, shortness of breath, and difficulty breathing during the treatment.
2. Do not skip any doses and complete the course as suggested by your doctor.
3. Use an effective method of birth control to avoid pregnancy while taking this medication.
4. Your doctor may get regular blood tests done to monitor your blood cell count (CBC) and electrolyte levels. liver and kidney function.
5. It is advisable to drink plenty of water to stay hydrated while taking this medicine.
6. Inform your doctor if you experience chills, fever, shortness of breath, and difficulty breathing during the treatment.
Warning, Precautions, and Side Effects Associated with Nimotuzumab
Warnings
Nimotuzumab may cause infusion reactions, such as fever, chills, nausea, vomiting, or difficulty breathing. These reactions typically occur during or shortly after the infusion, and your healthcare provider will monitor you closely during treatment. It may also lead to skin reactions like rash, itching, or dry skin. In rare cases, it can cause severe skin conditions like Stevens-Johnson syndrome or toxic epidermal necrolysis. Some patients may experience allergic reactions, which could include difficulty breathing, hives, or swelling of the face or throat. Nimotuzumab may also reduce white blood cell or platelet counts, increasing the risk of infection or bleeding. Additionally, it can cause pulmonary embolism, blood clots, and an elevated risk of heart attack.Precautions
Before starting nimotuzumab, it is essential to inform your healthcare provider about any past allergic reactions to medications or other substances, as well as any medications or supplements you are currently taking. This medication may lower white blood cell or platelet counts, increasing the risk of infection or bleeding, so your healthcare provider may monitor your blood cell counts during treatment. Additionally, nimotuzumab can harm an unborn baby or pass into breast milk, so women of reproductive age should avoid pregnancy and breastfeeding while taking this medication. If you have any concerns or questions about using nimotuzumab, be sure to discuss them with your healthcare provider.Side Effects
Like all medications, nimotuzumab can cause side effects, which can vary in severity and frequency between individuals. Common side effects include infusion reactions such as fever, chills, nausea, and vomiting, as well as skin reactions like rash, itching, and dry skin. Other common side effects are fatigue, weakness, headache, changes in taste or appetite, and pain or swelling at the injection site. In rare cases, nimotuzumab may cause more serious side effects, such as an allergic reaction with symptoms like difficulty breathing, hives, or swelling of the face or throat, as well as a decrease in white blood cells or platelets. Severe skin reactions are also possible.Word of Advice
It is essential to carefully read the medication label before taking Nimotuzumab and follow the instructions provided by both your healthcare provider and the label, including dosage and frequency. Your healthcare provider can offer more information on the risks and benefits of the medication and help determine if it is the right treatment for you. Be sure to report any side effects experienced during treatment to your healthcare provider. Pregnancy and breastfeeding are not recommended while taking this medication. Before starting Nimotuzumab or any other medication, inform your healthcare provider of any allergies or medications you are taking to help avoid potential interactions or adverse effects.FAQ - Nimotuzumab
1Is Biomab EGFR 50mg Injection used for cancer prevention?
Currently, Biomab EGFR 50mg Injection is approved only for cancer treatment, not prevention. However, ongoing research is exploring its potential role in cancer prevention and early detection.
2Can Biomab EGFR 50mg Injection cure cancer?
Biomab EGFR 50mg Injection is not a cure for cancer, but it can be an effective treatment for certain types of solid tumors. Its effectiveness may vary based on the stage and type of cancer, as well as individual factors.
3Is it possible to combine Biomab EGFR 50mg Injection with other cancer treatments?
Yes, Biomab EGFR 50mg Injection is commonly used alongside other cancer treatments, such as chemotherapy and radiation therapy. Your doctor will decide on the most suitable treatment plan based on your specific condition.
4Is Biomab EGFR 50mg Injection capable of curing cancer?
Biomab EGFR 50mg Injection is not a cure for cancer, but it can be an effective treatment for certain types of solid tumors. Its effectiveness may vary based on the cancer's stage and type, as well as individual factors.
5What is the shelf life of Biomab EGFR 50mg Injection?
The shelf life of Biomab EGFR 50mg Injection may vary based on the manufacturer and storage conditions. It is essential to store the medication as instructed and verify the expiration date before use.
6Can Biomab EGFR 50mg Injection cause hair loss?
Hair loss is not a typical side effect of Biomab EGFR 50mg Injection. However, certain chemotherapy drugs that are commonly used alongside this medication may cause hair loss as a side effect.
7Are there any foods I should avoid while taking Biomab EGFR 50mg Injection?
There are no specific foods you need to avoid while taking Biomab EGFR 50mg Injection. However, it is important to maintain a healthy, balanced diet to support your overall health and well-being during cancer treatment.
References
1. Bespons Pvt Ltd, U.S. Food and Drug Administration., Highlights of prescribing information - Biomab EGFR 50mg Injection, [Last updated: March 2020],[10 April 2023], Available at:https://www.accessdata.fda.gov/
drugsatfda_docs/label/2017/761040s000lbl.pdf
2. S.H. Ibbotson, R.S. Dawe, Skin Disease, Davidson’s Principles & Practice of Medicine, 22nd Edition, 2014, 1249-1305
3. Goodman & Gilman's The Pharmacological Basis of Therapeutics (Brunton, L.L., Chabner, B.A., and Knollmann, B.C., eds.). Biomab EGFR 50mg Injection. 13th edition, 1461-1463.
4. KD Tripathi, Essentials of Medical Pharmacology, 7th edition, Jaypee Brothers Medical Publishers (P) Ltd.; 2013, 893-894.
drugsatfda_docs/label/2017/761040s000lbl.pdf
2. S.H. Ibbotson, R.S. Dawe, Skin Disease, Davidson’s Principles & Practice of Medicine, 22nd Edition, 2014, 1249-1305
3. Goodman & Gilman's The Pharmacological Basis of Therapeutics (Brunton, L.L., Chabner, B.A., and Knollmann, B.C., eds.). Biomab EGFR 50mg Injection. 13th edition, 1461-1463.
4. KD Tripathi, Essentials of Medical Pharmacology, 7th edition, Jaypee Brothers Medical Publishers (P) Ltd.; 2013, 893-894.