Rucaparib
This page provides concise information on the drug rucaparib, including its indications, dosage and administration details, mechanism of action, associated brands with strengths, warnings, and prevalent side effects.
Context and Approval Date
Rucaparib is an anticancer drug that belongs to the category of PARP (poly ADP-ribose polymerase) inhibitors and received FDA approval on December 19, 2016. It is prescribed for the treatment of specific types of cancer.
Mechanism of Action of Rucaparib
Rucaparib stops polyadenosine diphosphate-ribose polymerase enzymes from working. These enzymes help fix DNA and are called PARP-1, PARP-2, and PARP-3. It blocks an enzyme that repairs damaged DNA in the cancer cells, resulting in their death.
All Substitutes
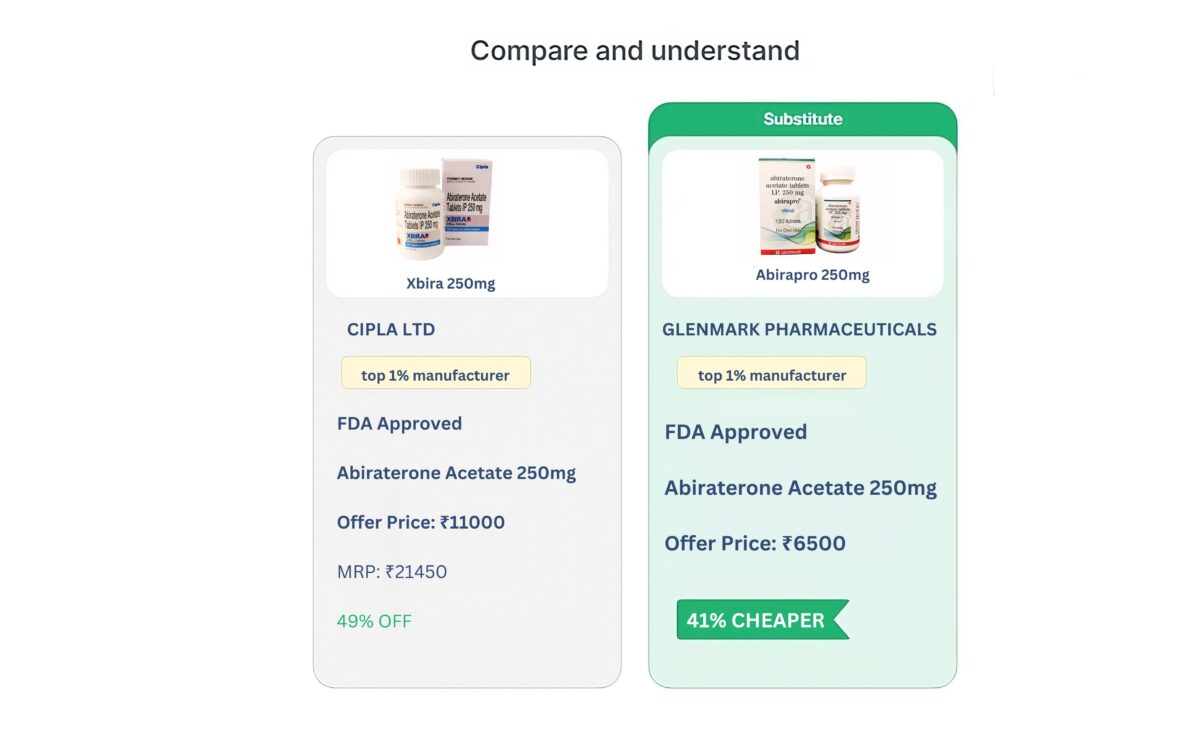
View All| Product | Packaging Size | Company | Price |
|---|---|---|---|
| Bdparib 300mg tablet | 60 Tablets | BDR Pharmaceuticals | INR 29000 |
| Nuparp 300mg tablet | 60 Tablets | Zydus Cadila | INR 24500 |
| Rucaza 300mg tablet | 60 Tablets | Intas Pharmaceuticals Ltd | INR 27000 |
| Ruparib 300mg tablet | 60 tablets | Emcure Pharmaceuticals Ltd | INR 22500 |
| Lucaparib 300mg tablet | 60 Tablets | Lupin Ltd | INR 38000 |
| Nuparp 200mg tablet | 60 Tablets | Zydus Cadila | INR 22000 |
Uses of Rucaparib
As an adult, rucaparib can help other chemotherapy treatments work better for people with certain types of ovarian cancer, fallopian tube cancer, and recurrent primary peritoneal cancers that have had partial or full responses to those treatments in the past.
Additionally, it is employed in the treatment of particular cancers in individuals with specific gene (BRCA) alterations who have undergone at least two other chemotherapy regimens. Furthermore, it is indicated for maintenance therapy directly following chemotherapy that has resulted in tumor shrinkage.
Additionally, it is employed in the treatment of particular cancers in individuals with specific gene (BRCA) alterations who have undergone at least two other chemotherapy regimens. Furthermore, it is indicated for maintenance therapy directly following chemotherapy that has resulted in tumor shrinkage.
Rucaparib administration guidelines and available dosage options
This is an orally administered antineoplastic medication, provided in tablet form at 200mg, 250mg, and 300mg. Your doctor will decide on the appropriate rucaparib dose and duration taking into account your unique medical situation and other pertinent factors. It is crucial to adhere to your doctor's prescription instructions. Take the tablet at a consistent time, with or without food, and ensure not to break, chew, crush, or split the tablet. Swallow the entire tablet with water.
Information about rucaparib
Rucaparib uses
. Prostate cancer. Ovarian cancer
How rucaparib works
Rucaparib prevents the repair of damaged DNA in cancer cells by blocking specific proteins, leading to their death.Common side effects of rucaparib
Nasopharyngitis (inflammation of the throat and nasal passages), Rash, Increased liver enzymes, Decreased appetite, Anemia (low number of red blood cells), Weakness, Gastrointestinal disturbance, Myelodysplastic syndrome, acute myeloid leukemia (blood cancer).1. Before taking Rucaparib, make sure you tell your doctor about any other medications you are taking (including prescription, over-the-counter, vitamins, herbal remedies, etc.).
2. After starting your treatment with Rucaparib, you may be at risk of infection, so try to avoid crowds or people with colds. Report fever or any other signs of infection immediately to your doctor.
3. Drink at least two to three-quarters of fluid every 24 hours, unless you are instructed otherwise.
4. Take the antinausea medications your doctor has prescribed, and eat small, frequent meals, to reduce nausea.
5. Females who are able to become pregnant should use effective birth control during the treatment and for at least 6 months after receiving the last dose of rucaparib.
6. Avoid breastfeeding during the treatment with Rucaparib and for 2 weeks after the last dose.
7. If you are a male with a female partner who is pregnant or able to conceive, use effective birth control (condom) during the treatment and for 3 months after the last dose of Rucaparib.
2. After starting your treatment with Rucaparib, you may be at risk of infection, so try to avoid crowds or people with colds. Report fever or any other signs of infection immediately to your doctor.
3. Drink at least two to three-quarters of fluid every 24 hours, unless you are instructed otherwise.
4. Take the antinausea medications your doctor has prescribed, and eat small, frequent meals, to reduce nausea.
5. Females who are able to become pregnant should use effective birth control during the treatment and for at least 6 months after receiving the last dose of rucaparib.
6. Avoid breastfeeding during the treatment with Rucaparib and for 2 weeks after the last dose.
7. If you are a male with a female partner who is pregnant or able to conceive, use effective birth control (condom) during the treatment and for 3 months after the last dose of Rucaparib.
Warning, Precautions, and Side Effects Associated with Rucaparib
Warning
This medication can cause decreased levels of red blood cells, white blood cells, or platelets, showing up as symptoms like fever, infection, bruising, or bleeding. A reduced blood cell count may suggest bone marrow issues such as myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML). Your doctor may advise regular blood tests to keep an eye on these levels before and during the course of treatment. It's essential to note that Rucaparib may pose a risk of fetal harm, so inform your physician if you are pregnant or planning pregnancy during the treatment.Precautions
Notify your physician if you experience nausea, vomiting, diarrhea, or abdominal pain during the course of treatment, as this medication may impact your stomach or bowels. Rucaparib is not recommended for individuals under 18 years of age. Avoid using this medicine if you have an allergy to rucaparib or any of its components. For female patients of reproductive age, use effective contraception throughout the treatment and for 6 months following the final dose. Male patients with female partners of reproductive potential should use effective contraception during the treatment and for 3 months after the last dose.Side Effects
Common adverse effects associated with rucaparib include rash, constipation, reduced appetite, diarrhea, indigestion, nausea, vomiting, alterations in blood count, shortness of breath, and fatigue. Additionally, severe anemia, hypersensitivity reactions, sepsis (blood infection), and pneumonia are among the more serious potential side effects.Word of Advice
Store this medication at temperatures between 20°C and 25°C, protecting it from light and excess moisture. Ensure it is kept out of the reach of children and pets. In case of a missed tablet, take the next dose at the designated time; avoid doubling up to compensate for a missed dose. Inform your healthcare provider about all medications, including prescriptions, over-the-counter drugs, nutritional supplements, vitamins, and herbal products, as certain interactions may impact Rucaparib's efficacy, leading to undesired side effects.Exercise caution in sunlight during Rucaparib treatment, as sunburn may occur easily. Stay out of direct sunlight, wear protective clothing covering your head, arms, and legs, and use sunscreen and lip balm with a suitable SPF after consulting your doctor. If vomiting occurs post-dose, refrain from taking an additional dose; stick to the regular schedule for the next dose. Do not discontinue this medication without consulting your doctor, who may conduct lab tests to assess treatment effectiveness and monitor potential adverse effects.
FAQ - Rucaparib
1Is the administration of Rucaparib considered safe for patients with kidney disease?
Absolutely, the administration of Rucaparib is safe for patients with kidney disease, and there is no need for dose adjustments. It is advisable to inform your physician about any existing kidney disorders before commencing the therapy.
2Can one safely operate a vehicle after taking Rucaparib?
The potential for dizziness and drowsiness with rucaparib exists. It is unsafe to drive or operate heavy machinery if you encounter any symptoms that compromise your ability to concentrate and react.
3What information should I provide to my healthcare provider prior to taking Rucaparib?
Notify your doctor about your complete medical and medication history, including over-the-counter medications, as well as any nutritional or vitamin supplements you are currently using.
4As a male patient, is it necessary to practice contraception during the course of this medication?
Absolutely, male patients with female partners of reproductive potential should use effective contraception throughout the treatment and for three months following the last dose of rucaparib.
5Is it necessary to use sunscreen while undergoing treatment with rucaparib?
Actually, this medication heightens skin sensitivity to sunlight. Consult your doctor and, if advised, use sunscreen during the treatment.
6What is the duration of the Rucaparib treatment?
The duration of your treatment depends on the nature and severity of your disease. Your doctor will determine both the dosage and the length of your treatment.
References
1. HIGHLIGHTS OF PRESCRIBING INFORMATION - Rucaparib. [revised on 2018] [cited 2023 Apr 11]. Available from: https://www.accessdata.fda.gov/
drugsatfda_docs/label/2018/209115s003lbl.pdf
2. Rubraca 250mg film-coated tablets - Summary of Product Characteristics (SmPC) - (emc). www.medicines.org.uk. [revised on 2022] [cited 2023 Apr 11]. Available from: https://www.medicines.org.uk/emc/product
/10027/smpc
3. Shirley M. Rucaparib: A Review in Ovarian Cancer. Targeted Oncology. [2019 Apr 1] [cited 2022 Oct 15];14(2):237–46. Available from: https://pubmed.ncbi.nlm.nih.gov/30830551/
drugsatfda_docs/label/2018/209115s003lbl.pdf
2. Rubraca 250mg film-coated tablets - Summary of Product Characteristics (SmPC) - (emc). www.medicines.org.uk. [revised on 2022] [cited 2023 Apr 11]. Available from: https://www.medicines.org.uk/emc/product
/10027/smpc
3. Shirley M. Rucaparib: A Review in Ovarian Cancer. Targeted Oncology. [2019 Apr 1] [cited 2022 Oct 15];14(2):237–46. Available from: https://pubmed.ncbi.nlm.nih.gov/30830551/